
Patrick J. Kelly, M.D., FACDS
Professor and Chairman
Department of Neurological Surgery
New York University Medical Center
550 First Ave
New York, N.Y. 10016
email: surg@mcns04.med.nyu.edu
The astrocytoma is derived from a normal supporting cell in the brain called the astrocyte. In a patient with one of these tumors, the cells in the astrocytoma tumor are no longer normal; and the degree of this abnormality is used to determine the tumor's grade. The tumor's grade determines the prognosis of the tumor. Astrocytomas are graded from 1 to 4, with grade 1 being the slowest growing and grade 4 being the most rapidly growing and malignant lesions. The following descriptions refer to the appearance of the tumor under the pathologist's microscope.
Grade 1: In these tumors astrocytic tumor cells are usually normal in appearance except that there are more of them than normally seen in microscopic examinations of brain tissue. Usually grade 1 astrocytomas produce epileptic seizures as their only symptom since their presence is irritating to surrounding brain tissue. They can also become quite large since they are well tolerated by the brain. However, when the mass effect of the tumor and the mass of the brain combine within the non-yielding skull cavity; a rise in pressure inside the skull results. This can cause headaches, paralysis, personality change, coma and death. The prognosis for grade 1 astrocytomas is generally good. Sometimes surgery to reduce mass effect is required, however. Patients with grade 1 astrocytomas have been known to live 30 years or more following diagnosis. Radiation therapy is probably not appropriate in these tumors. The CT and MRI appearance of these lesions is shown in
Figure 1a,b
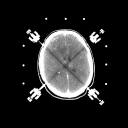 (44Kb) (CT)
(44Kb) (CT) 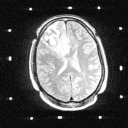 (137Kb) (MRI).
(137Kb) (MRI).
Pilocytic astrocytomas: These benign astrocytomas tend to occur in children and young adults, are histologically circumscribed . Despite the fact that many are located in the thalamus and other important subcortical locations, they can be completely resected by computer assisted stereotactic technique with excellent postoperative results. These lesions exhibit prominent enhancement on CT or on MR imaging with gadolinium (as shown in Figures 2A and 2B). The histologic borders are usually defined accurately by the contrast enhancement.
Figure 2a,b
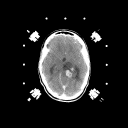 (44Kb) (CT)
(44Kb) (CT) 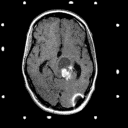 (137Kb) (MRI)
(137Kb) (MRI)
Grade 2: In grade 2 tumors, tumor cells are slightly abnormal in appearance as well as increased in number. The variations in appearance of these cells is referred to as pleomorphism. There should be no mitotic figures (indications that the cells are dividing) and no necrosis (dead tissue). In general, these tumors are made up of isolated tumor cells within functioning brain tissue. On imaging studies these lesions show hypodensity on CT and prolongation of T1 and T2 on MRI as shown in Figures 3A and 3B. They only very rarely exhibit contrast enhancement.
Figure 3a,b,c
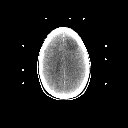 (37Kb) (CT)
(37Kb) (CT) 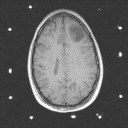 (119Kb) (MRI).
(119Kb) (MRI). 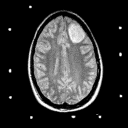 (60Kb) (MRI).
(60Kb) (MRI).
Removal of the tumor is, in fact, removal of this "sick" brain tissue. These tumors are, therefore, usually biopsied only; unless they are located in unimportant brain tissue- in which case they can be removed ( as in the case shown in Figure 3). In addition, these lesions (tumors) rarely produce paralysis.
There remains some debate on the place for radiation therapy and chemotherapy in these tumors. However, recent studies have shown that 5 year survival in grade 2 astrocytomas without treatment is about 34%; and with treatment (radiation therapy): about 70%. Therefore most centers recommend radiation therapy after a grade 2 astrocytoma is diagnosed by biopsy or some other surgical procedure.
Grade 3: These and Grade 4 astrocytomas are frequently referred to as malignant astrocytomas. They exhibit contrast enhancement on imaging studies. Frequently, the contrast enhancing mass is surrounded by a zone of hypodensity on CT and prolonged T1 and T2 on MRI as shown in Figure 4. This zone is frequently called "edema" and it is edematous brain parenchyma infiltrated by isolated tumor cells.
Figure 4a,b,c
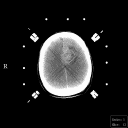 (34Kb) (CT)
(34Kb) (CT) 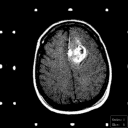 (54Kb) (MRI)
(54Kb) (MRI) 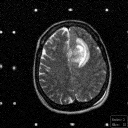 (117Kb) (MRI)
(117Kb) (MRI)
In another classification scheme these are referred to as anaplastic astrocytomas. In grade 3 tumors, cells are not only abnormal in appearance but some show evidence of mitosis. Mitosis is the cellular process by which cells divide; where one cell becomes two. Mitoses are apparent to the pathologist as the surgical specimen is reviewed under the microscope. Some of the cells in the tumor infiltrate into brain tissue- similar to the picture seen with grade 1 and grade 2 astrocytomas; other cells stay put and continue to divide and destroy the brain parenchyma in which they reside as the joined cells for a mass of solid tumor tissue. When the tumor tissue is formed in important brain areas, neurological deficits corresponding to that area result because the brain tissue in that area is destroyed by the evolving tumor tissue mass. For example, a grade 3 astrocytoma forming in the central area of the brain, with formation of solid tumor tissue in the motor area will produce weakness and paralysis on the opposite side of the patient's body ( remember that the left side of the brain controls the right side of the body and vice versa).
Treatment for grade 3 astrocytomas involves establishing the diagnosis by surgery or stereotactic biopsy and follow-up with radiation therapy and chemotherapy. The average survival of patients with grade 3 astrocytomas is 18 months with treatment.
Grade 4: Grade 4 astrocytomas ( frequently referred to as glioblastomas or glioblastoma multiforme) are the most malignant variety of these tumors. They are made up of cells which infiltrate brain tissue with a region (and in some cases regions) of solid tumor tissue within the zone of infiltrated brain tissue. Mitoses are frequently noted by the pathologist as the surgical specimen is examined. In addition, regions of necrosis (dead tissue) are also noted- where the tumor has grown so fast that parts of it has outpaced its blood supply. These tumors induce the formation of new but abnormal blood vessels which when identified are also important in establishing the diagnosis. The CT and MRI demonstrate a contrast enhancing mass with a hypodense center (which corresponds to necrosis) surrounded by a zone of hypodensity on CT and prolonged T1 and T2 on MRI which corresponds to infiltrated parenchyma as shown in Figure 5.
Figure 5a,b,c
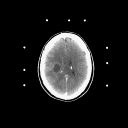 (35Kb) (CT)
(35Kb) (CT) 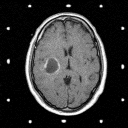 (97Kb) (MRI)
(97Kb) (MRI) 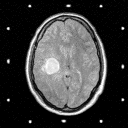 (94Kb) (MRI)
(94Kb) (MRI)
The grade 4 astrocytoma has the worst prognosis of all: 17 weeks average (mean) survival after diagnosis without treatment; 30 weeks average survival with biopsy followed by radiation therapy; 37 weeks average survival following surgical removal of most of the tumor tissue component of the tumor and radiation therapy and 51 weeks average survival following stereotactic volumetric resection of the tumor tissue component and radiation therapy. The prognosis for any patient with a malignant astrocytoma (grade 3 or 4) is also very dependent upon age (older people do not live as long as young patients) and performance status ( patients who are neurologically normal and independent live longer than patients who have a neurological deficit). Chemotherapy has been shown to add several weeks on to the survival. Radiation implants (brachytherapy) have also been shown to increase survival but more than half of these patients require another operation to remove dead tissue resulting from the radiation.
There are many experimental therapies- none of which have been shown to be curative yet. These include brachytherapy (stereotactic interstitial irradiation), stereotactic radiosurgery (focused one shot high dose irradiation to the tumor), immunotherapy ( where lymphocytes conditioned to attack brain tumor cells are injected into the tumor or cavity made by surgical removal of part of the tumor) and most recently gene therapy ( where the brain is infected by a genetically engineered virus which attacks tumor cells). Clinical trials are underway for the evaluation of all of these.